This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Vyhledávání
Atomic absorption spectrometry (AAS)
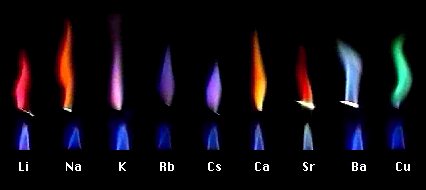
Atomic absorption spectrometry (AAS) is a method of quantitative analysis which is based on the absorption of the radiation energy by free atoms for the determination of the content of individual elements in a sample with high accuracy (hundredths to hundreds of ppm). It should be noted that AAS is a comparative method that compares the measured values with a reference value given for a specific element. The sample of the material in the form of aerosol is supplied to the burner where the heat of the flame triggers atomization. The flame is then passed through with a radiation beam, causing the beam to weaken. This weakening, typical of a given element, corresponds to the concentration of the element in the material.
Absorption environment
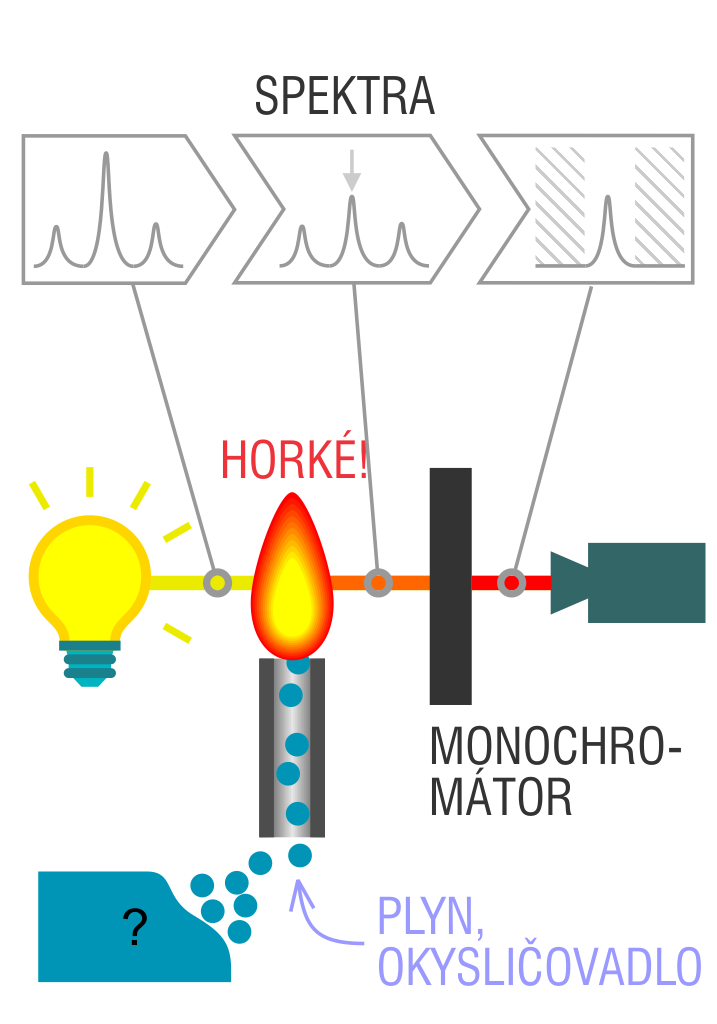
The entire method is based on the transformation of a sample into atomic state. Most frequently, a flame is used for atomic state transformation. The sample, typically in the form of a solution (a powder can be used as well) is supplied to a nebulizer, and the resulting aerosol is fed to the burner, where a mixture of gas and oxidizer (typically air-acetylene, or acetylene and nitrous oxide for hard-to-atomize elements) is already burning. By mixing both gases in specified ratios, we obtain either a reducing or oxidizing flame, selecting the right one depending on its suitability for the atomization of a specific element. The mouth of the burner is shaped as a thin slit of various lengths, depending on the type of the gas mixture used.
The slit length defines the maximum achievable thickness of the absorption environment layer, which the radiation is passing through. The composition and the temperature of the flame varies with its height. Therefore, there are optimal zones for each element in the flame, defined as the height above the burner mouth, where the free-atom concentration is the highest. The flame height needs to be determined experimentally. For this reason, the position of the burner must be adjustable in the horizontal as well as vertical direction. Then, the aerosol vaporization and subsequent atomization of the analyte are triggered by the flame, creating the absorption environment; i.e. free electrons of the studied sample are in ground state.
Through the cloud of atoms, radiation is passed from a source that generates such wavelenghts that can be absorbed by the atoms of the element being determined. This radiation corresponds to the radiation of the atoms of the element (for each element to be determined “its” separate lamp – a vacuum tube – must be used). The absorption weakens the radiation flux.
Monochromator and detector
The next one to follow in the line is a grating monochromator used for isolating the radiation of suitable wavelength. The tilt of the grating sets the wavelenght of a resonance line to a maximum throughput. The respective width of the spectral interval, regulated by input and output slits of the monochromator, is selected to ensure that the detector is hit exclusively with resonance radiation and not with any non-absorbing radiation of the lines of the close wavelenghts.
The next in the line after the output slit is a photomultiplier with a photocathode, whose sensitivity is sufficient for the spectral area in observation; it is used to detect radiation. The detector is equipped with a logarithmic converter for direct reading of absorbance values. Absorbance values are deduced in analogue or digital form.

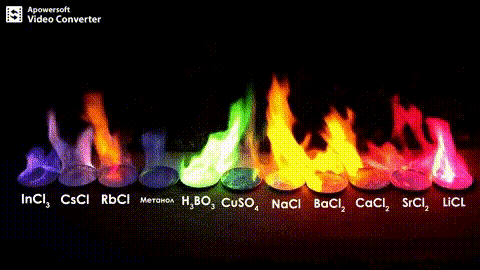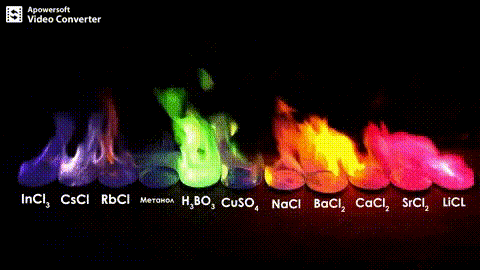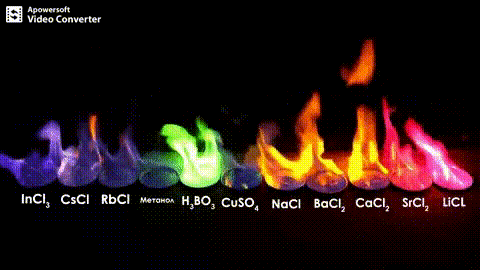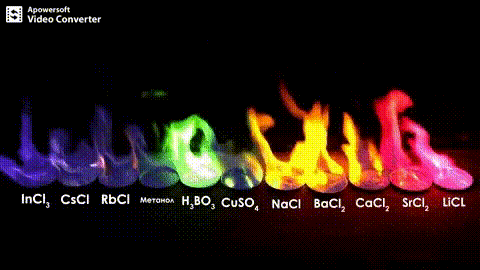
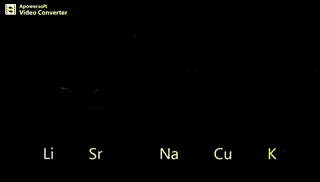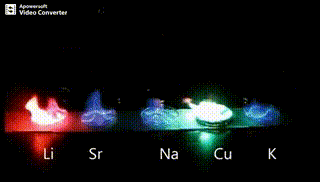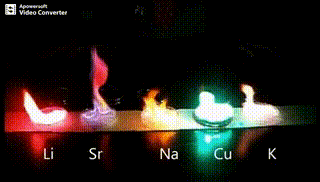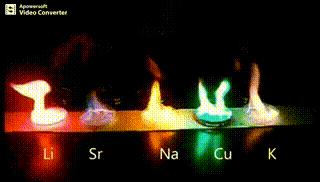
 The NCK for MATCA is supported by the
The NCK for MATCA is supported by the