This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Vyhledávání
Glow Discharge Optical Emission Spectroscopy (GDOES)
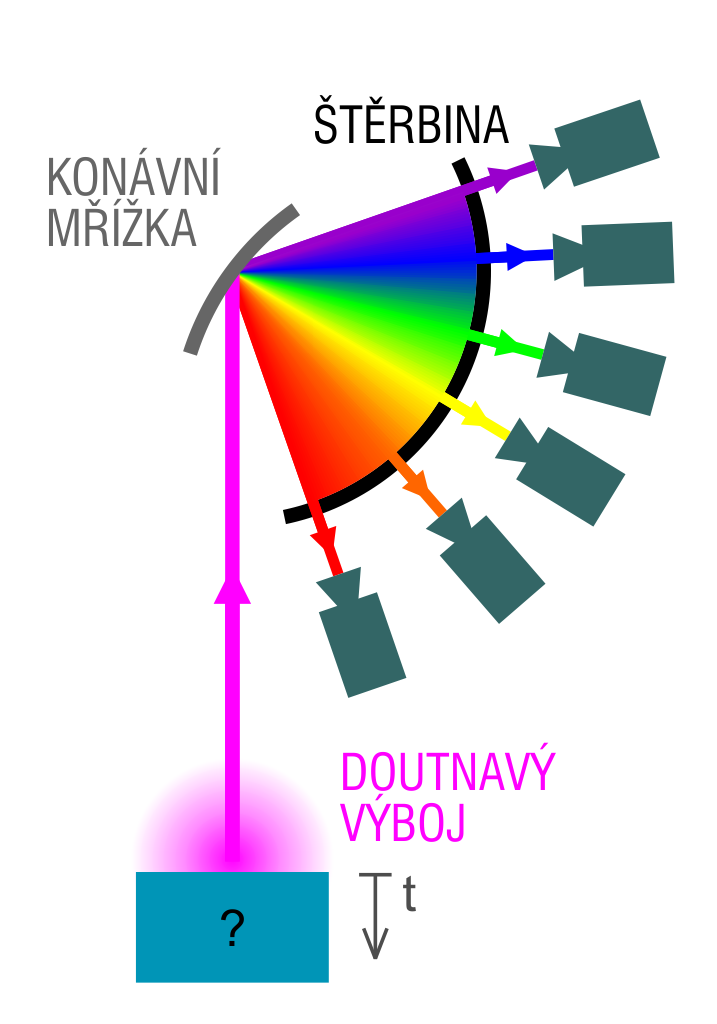
Optical chemical spectroscopy is one of the classic methods of direct chemical analysis of solid samples, that is, without converting the sample into a solution. The emission spectra are excited using different types of electric charges such as spark discharge, electric arc or glow charge. We are going to deal with glow discharge optical emission spectroscopy, known as the GD-OES.
To be able to carry out a GD-OES analysis, a sample with a plane is required, whose linear dimension is at least several millimetres. The sample is placed into an excitation source called Grimm lamp, in which it is conductively connected to a cathode, separating the inside of the vacuum tube from the atmosphere. The vacuum tube gets exhausted inside, then it is filled with a working gas, typically argon, and under the working pressure in the order of hundreds of Pa, high-voltage is turned on (typically 700-1200 V).
Inside the vacuum tube in the anode cavity, a glow discharge is initiated, where the analysed sample acts as a cathode. Typical current densities use the range between 200 and 500mA/cm2, the anode diameter is usually 4 mm. Heavy argon ions from the glow discharge plasma are accelerated by an electrical field in the direction of the sample surface, which they hit with high energy, knocking out atoms that were originally bound in the crystal grid of the sample. In this process, the knocked out atoms appear in the area of the plasma with high concentration of free electrons, where they get excited primarily in collisions with electrons, emitting characteristic radiation during de-excitation. This radiation is an atomic emission spectrum consisting of the spectra of individual constituent elements. This spectrum is analysed with optical spectrometer.
The atomization method described above is called cathode sputtering, and its important property is that the individual surface layers of the sample are being eliminated one after another in the order in which they are layered (in the downward direction). So if we are considering the signals from individual detectors as a function of time, we are getting a piece of information about the ordering of the respective element depending on the depth under the surface. This is how we can measure the concentration-depth profile of all the elements present in the sample. Typical sputtering speeds are in the order of several micrometers per minute, depending on the electric conditions of the charge and the type of the analysed material.

 The NCK for MATCA is supported by the
The NCK for MATCA is supported by the