This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Vyhledávání
Raman spectroscopy
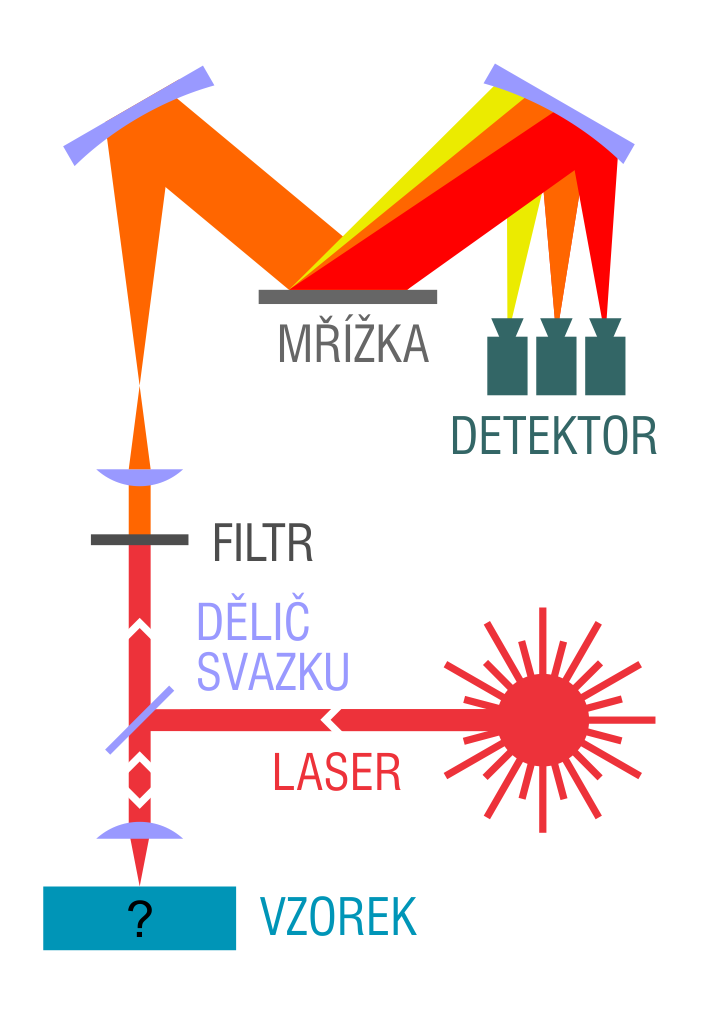
Raman effect is an inelastic scattering of light on particles (molecules and atoms), during which the particles transition to one of the quantum states by releasing an energy quantum. Raman spectroscopy is a very effective and non-destructive technique, which provides information about vibrational and rotational transitions in molecules. It is useful in a number of applications, including basic research, routine process management, and material identification.
The molecule first absorbs a photon of the exciting radiation (it is excited from a ground state into a virtual energy state). Then, during relaxation, the molecule emits a photon and transitions into the final (vibrational or rotational) state, which is not identical to the ground energy state of the molecule. The energy difference for the emitted electrons between the ground and final state leads to a frequency shift away from the exciting frequency.
If the final vibrational state corresponds to a higher (or lower) energy value than the ground state, then the emitted photon will be shifted towards lower (or higher) frequencies. Accordingly, the Raman scattering spectrum consists of a pair of lines, which are distributed symmetrically relative to the line of the elastically scattered radiation of the ω0 frequency (see image). The region of lower frequencies is called the Stokes branch, whereas the higher frequency region is called the anti-Stokes branch of the Raman spectrum. In practice, we typically measure the Stokes branch of the Raman spectrum as it has a considerably higher intensity.
The Raman scattering spectrum of a substance is very weak in intensity, which is very impractical for a number of measurements. This is one of the reasons why the special Raman spectroscopy techniques are becoming increasingly more popular as they can overcome certain limitations posed by the standard Raman effect. If the wavelenght of the excitation laser used corresponds to the energy which is needed for the transition of an electron in the molecule of the studied substance to a higher energy level, the Raman effect resonance amplification by 2 to 4 orders may occur. Each type of molecular bond corresponds to a different wavelenght. Then, this is called a selective resonance Raman scattering.
Surface–Enhanced Raman Scattering (SERS) means a significant Raman scattering amplification as a result of an interaction between the visible light and plasmonic metal nanostructures and molecules localized on their surfaces. The studied molecules are first absorbed on a suitable metallic substrate. Most frequently, we use SERS substrates containing golden or silver nanoparticles. The overall amplification of Raman scattering intensity is enhanced by two mechanisms: the electromagnetic effect (resonant excitation of surface plasmons in a metal), and the chemical effect (charge transfer between an absorbate and metallic surface).

 The NCK for MATCA is supported by the
The NCK for MATCA is supported by the